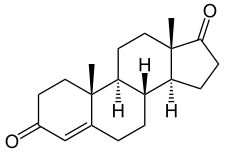
Androstenedione
 | |
 | |
| Clinical data | |
|---|---|
| Other names | A4; Δ4-dione; Androstenedione; Androst-4-ene-3,17-dione; 4-Androstene-3,17-dione; 17-Ketotestosterone; 17-Oxotestosterone; 3,17-Dioxoandrost-4-ene; Fecundin |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.504 |
| Chemical and physical data | |
| Formula | C19H26O2 |
| Molar mass | 286.415 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.11±0.1 [1] g/cm3 (predicted) at 20°C and 760 Torr. Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs). |
| Melting point | 173–174[2] °C (343–345 °F) |
| |
| |
| (verify) | |
Androstenedione, or 4-androstenedione (abbreviated as A4 or Δ4-dione), also known as androst-4-ene-3,17-dione, is an endogenous weak androgen steroid hormone and intermediate in the biosynthesis of estrone and of testosterone from dehydroepiandrosterone (DHEA). It is closely related to androstenediol (androst-5-ene-3β,17β-diol).
Function
[edit]Androstenedione is a precursor of testosterone and other androgens, as well as of estrogens like estrone, in the body. In addition to functioning as an endogenous prohormone, androstenedione also has weak androgenic activity in its own right.
Androstenedione has been found to possess some estrogenic activity, similarly to other DHEA metabolites.[3] However, in contrast to androstenediol, its affinity for the estrogen receptors is very low, with less than 0.01% of the affinity of estradiol for both the ERα and ERβ.[4]
Adrenarche
[edit]In children aged 6 to 8 years old, there is a rise in androstenedione secretion along with DHEA during adrenarche. This rise in androstenedione and DHEA is hypothesized to play a crucial role for learning social, cultural and ecological skills, such as the development and understanding of sexual attraction. Furthermore, it is thought that androstenedione plays a role in levels of aggression and competition in boys, as a positive correlation between the two were observed, while testosterone levels were below detection.[5]
Biochemistry
[edit]Biosynthesis
[edit]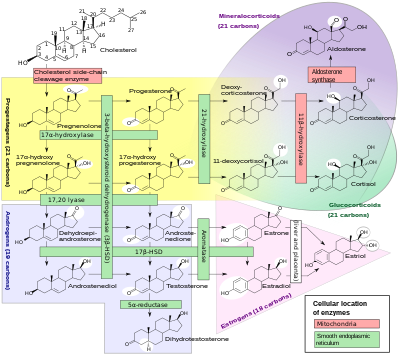
Androstenedione is the common precursor of the androgen and estrogen sex hormones.[7]
Androstenedione can be biosynthesized in one of two ways. The primary pathway involves conversion of 17α-hydroxypregnenolone to DHEA by way of 17,20-lyase, with subsequent conversion of DHEA to androstenedione via the enzyme 3β-hydroxysteroid dehydrogenase. The secondary pathway involves conversion of 17α-hydroxyprogesterone, most often a precursor to cortisol, to androstenedione directly by way of 17,20-lyase. Thus, 17,20-lyase is required for the synthesis of androstenedione, whether immediately or one step removed.
Androstenedione is produced in the adrenal glands and the gonads. The production of adrenal androstenedione is governed by adrenocorticotrophic hormone (ACTH), whereas production of gonadal androstenedione is under control by the gonadotropins. In premenopausal women, the adrenal glands and ovaries each produce about half of the total androstenedione (about 3 mg/day). After menopause, androstenedione production is about halved, due primarily to the reduction of the steroid secreted by the ovary. Nevertheless, androstenedione is the principal steroid produced by the postmenopausal ovary.
Some androstenedione is also secreted into the plasma, and may be converted in peripheral tissues to testosterone and estrogens.
| Sex | Sex hormone | Reproductive phase |
Blood production rate |
Gonadal secretion rate |
Metabolic clearance rate |
Reference range (serum levels) | |
|---|---|---|---|---|---|---|---|
| SI units | Non-SI units | ||||||
| Men | Androstenedione | –
|
2.8 mg/day | 1.6 mg/day | 2200 L/day | 2.8–7.3 nmol/L | 80–210 ng/dL |
| Testosterone | –
|
6.5 mg/day | 6.2 mg/day | 950 L/day | 6.9–34.7 nmol/L | 200–1000 ng/dL | |
| Estrone | –
|
150 μg/day | 110 μg/day | 2050 L/day | 37–250 pmol/L | 10–70 pg/mL | |
| Estradiol | –
|
60 μg/day | 50 μg/day | 1600 L/day | <37–210 pmol/L | 10–57 pg/mL | |
| Estrone sulfate | –
|
80 μg/day | Insignificant | 167 L/day | 600–2500 pmol/L | 200–900 pg/mL | |
| Women | Androstenedione | –
|
3.2 mg/day | 2.8 mg/day | 2000 L/day | 3.1–12.2 nmol/L | 89–350 ng/dL |
| Testosterone | –
|
190 μg/day | 60 μg/day | 500 L/day | 0.7–2.8 nmol/L | 20–81 ng/dL | |
| Estrone | Follicular phase | 110 μg/day | 80 μg/day | 2200 L/day | 110–400 pmol/L | 30–110 pg/mL | |
| Luteal phase | 260 μg/day | 150 μg/day | 2200 L/day | 310–660 pmol/L | 80–180 pg/mL | ||
| Postmenopause | 40 μg/day | Insignificant | 1610 L/day | 22–230 pmol/L | 6–60 pg/mL | ||
| Estradiol | Follicular phase | 90 μg/day | 80 μg/day | 1200 L/day | <37–360 pmol/L | 10–98 pg/mL | |
| Luteal phase | 250 μg/day | 240 μg/day | 1200 L/day | 699–1250 pmol/L | 190–341 pg/mL | ||
| Postmenopause | 6 μg/day | Insignificant | 910 L/day | <37–140 pmol/L | 10–38 pg/mL | ||
| Estrone sulfate | Follicular phase | 100 μg/day | Insignificant | 146 L/day | 700–3600 pmol/L | 250–1300 pg/mL | |
| Luteal phase | 180 μg/day | Insignificant | 146 L/day | 1100–7300 pmol/L | 400–2600 pg/mL | ||
| Progesterone | Follicular phase | 2 mg/day | 1.7 mg/day | 2100 L/day | 0.3–3 nmol/L | 0.1–0.9 ng/mL | |
| Luteal phase | 25 mg/day | 24 mg/day | 2100 L/day | 19–45 nmol/L | 6–14 ng/mL | ||
Notes and sources
Notes: "The concentration of a steroid in the circulation is determined by the rate at which it is secreted from glands, the rate of metabolism of precursor or prehormones into the steroid, and the rate at which it is extracted by tissues and metabolized. The secretion rate of a steroid refers to the total secretion of the compound from a gland per unit time. Secretion rates have been assessed by sampling the venous effluent from a gland over time and subtracting out the arterial and peripheral venous hormone concentration. The metabolic clearance rate of a steroid is defined as the volume of blood that has been completely cleared of the hormone per unit time. The production rate of a steroid hormone refers to entry into the blood of the compound from all possible sources, including secretion from glands and conversion of prohormones into the steroid of interest. At steady state, the amount of hormone entering the blood from all sources will be equal to the rate at which it is being cleared (metabolic clearance rate) multiplied by blood concentration (production rate = metabolic clearance rate × concentration). If there is little contribution of prohormone metabolism to the circulating pool of steroid, then the production rate will approximate the secretion rate." Sources: See template. | |||||||
Metabolism
[edit]Androstenedione is converted to either testosterone or estrone. Conversion of androstenedione to testosterone requires the enzyme 17β-hydroxysteroid dehydrogenase. Androstenedione is released into the blood by theca cells. Conversion of androstenedione to estrone requires the enzyme aromatase. Androstenedione is a substrate for estrogen production in granulosa cells which produce aromatase. Thus, theca cells and granulosa cells work together to form estrogens.[8]
Androstanedione is a 5α-reduced metabolite of 4-androstenedione which serves as an intermediate in the biosynthesis of the androgen and neurosteroid androsterone.[9]
Androstenedione also has a variety of metabolites in different species. In cattle, androstenedione is converted into oestradiol-17 and epitestosterone.[10] In sheep, the molecule is transformed into the 17-epimer.[11] Androstenedione is converted into different metabolites in invertebrates. As is the case for Marisa cornuarietis, freshwater ramshorn snail, where androstenedione is converted into 5α-dihydrotestosterone (DHT) and testosterone (T) in male and into 5α-dihydroandrostenedione (DHA) in females.[12]
Levels
[edit]Levels are normally 30–200 ng/dL (1.0–7.0 nmol/L) in females and 40–150 ng/dL (1.4–5.2 nmol/L) in males.
In premature infants, serum androstenedione levels hover between 80 and 446 ng/dL. In full-term newborns, levels range from 20 to 290 ng/dL, and between 1 month and 1 year old, serum levels typically stay at less than 69 ng/dL.
Serum levels of androstenedione greater than or equal to 500 ng/dL may indicate the presence of an adrenal or gonadal tumor.[13]
Pharmacology
[edit]Androstenedione has been shown to increase serum testosterone levels over an eight-hour period in men when taken as a single oral dose of 300 mg per day, but a dose of 100 mg had no significant effect on serum testosterone.[14] However, serum levels of estradiol increased following both the 100 mg and 300 mg doses.[14] The study also reported that the serum level of estrogens and testosterone produced varied widely between individuals.[14]
A 2006 review paper summarized several studies that examined the effect of androstenedione on strength training.[15] At dosages of 50 mg or 100 mg per day, androstenedione had no effect on muscle strength or size, or on body fat levels.[15] One study used a daily dosage of 300 mg of androstenedione combined with several other supplements, and also found no increase in strength when compared to a control group that did not take the supplements.[15]
The review authors speculate that sufficiently high doses may indeed lead to increased muscle size and strength.[15] However, due to the federal ban on androstenedione supplements, it is difficult to carry out new research on its effects.[15] The review authors conclude that individuals should not use androstenedione supplements due to the lack of evidence of beneficial effects, the wide variation in individual responses to the supplement, and the risk of unknown side effects.[15] Side effects for women may include the development of male characteristics, clitoromegaly, voice deepening, hirsutism, abnormal menstrual cycles and abnormal bleeding, blood clots, and metabolic disruption based on a study following 10 healthy females administering 100 mg androstenedione.[16]
Medical use
[edit]Under the brand name Metharmon-F and in combination with sex steroids (pregnenolone, testosterone, estrone, androstenediol) and thyroid hormone (desiccated thyroid), androstenedione is or has been marketed for medical use in Thailand.[17]
Chemistry
[edit]Androstenedione, also known as androst-4-ene-3,17-dione, is a naturally occurring androstane steroid and a 17-ketosteroid. It is closely related structurally to androstenediol (A5; androst-5-ene-3β,17β-diol), dehydroepiandrosterone (DHEA; androst-5-en-3β-ol-17-one), and testosterone (androst-4-en-17β-ol-3-one), as well as to 5α-androstanedione (5α-androstane-3,17-dione) and estrone (estra-1,3,5(10)-triene-3-ol-17-one or 19-norandrost-1,3,5(10)-triene-3-ol-17-one).
History
[edit]Use as a supplement
[edit]Androstenedione was manufactured as a dietary supplement, often called andro or andros for short. Sports Illustrated credits Patrick Arnold with introducing androstenedione to the North American market.[18] Androstenedione supplements are credited with increasing testosterone levels, enhancing athletic performance, building body muscles, reducing fats, increasing energy, maintaining healthy RBCs, and increasing sexual performance.[16] Although, all of these effects have not been proven through scientific study. Androstenedione was legal and able to be purchased over the counter, and, as a consequence, it was in common use in Major League Baseball throughout the 1990s by record-breaking sluggers like Mark McGwire.[19]
Barry R. McCaffrey, in his capacity as director of the White House Office of National Drug Control Policy from 1996 to 2001, determined that androstenedione could not be classified as an anabolic steroid because there is no proof that it promotes muscle growth.[20]
Society and culture
[edit]Regulation
[edit]Androstenedione is banned by the World Anti-Doping Agency, and from the Olympic Games. The International Olympic Committee in 1997 banned androstenedione and placed it under the category of androgenic-anabolic steroids.[20] Androstenedione is banned by MLB, the NFL, USOC, NCAA, and by the NBA.[20]
On March 12, 2004, the Anabolic Steroid Control Act of 2004 was introduced into the United States Senate. It amended the Controlled Substance Act to place both anabolic steroids and prohormones on a list of controlled substances, making possession of the banned substances a federal crime. The law took effect on January 20, 2005. However, androstenedione was legally defined as an anabolic steroid, even though there is scant evidence that androstenedione itself is anabolic in nature. On April 11, 2004, the United States Food and Drug Administration banned the sale of androstenedione, citing that the drug poses significant health risks commonly associated with steroids. Androstenedione is currently banned by the U.S. military.[21]
References
[edit]- Citations
- ^ "Androstenedione". SciFinder. American Chemical Society.
- ^ "Androstenedione Compound Summary". PubChem. National Center for Biotechnology Information. U.S. National Library of Medicine.
- ^ Miller KK, Al-Rayyan N, Ivanova MM, Mattingly KA, Ripp SL, Klinge CM, Prough RA (January 2013). "DHEA metabolites activate estrogen receptors alpha and beta". Steroids. 78 (1): 15–25. doi:10.1016/j.steroids.2012.10.002. PMC 3529809. PMID 23123738.
- ^ Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (March 1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology. 138 (3): 863–70. doi:10.1210/endo.138.3.4979. PMID 9048584.
- ^ Gray PB, McHale TS, Carré JM (May 2017). "A review of human male field studies of hormones and behavioral reproductive effort". Hormones and Behavior. 91: 52–67. doi:10.1016/j.yhbeh.2016.07.004. PMID 27449532. S2CID 4243812.
- ^ Häggström M, Richfield D (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- ^ Devlin TM (2010). Textbook of Biochemistry: with Clinical Correlations (7th ed.). Hoboken, NJ: John Wiley & Sons. p. 432. ISBN 978-0-470-28173-4.
- ^ Boulpaep EL, Boron WF (2005). Medical Physiology: A Cellular and Molecular Approach (Updated ed.). Philadelphia, Pa.: Elsevier Saunders. p. 1155. ISBN 978-1-4160-2328-9.
- ^ Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M (2011). [s://www.ingentaconnect.com/content/ben/cpd/2011/00000017/00000002/art00008 "Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders"]. Current Pharmaceutical Design. 17 (2): 151–67. doi:10.2174/138161211795049589 (inactive 2024-10-23). PMID 21361868.
{{cite journal}}: CS1 maint: DOI inactive as of October 2024 (link) - ^ Möstl E, Choi HS, Bamberg E (1980). "Rapid conversion of androstenedione into epitestosterone in bovine blood invitro". IRCS Medical Science: Biochemistry. 8: 440.
- ^ Tsan CP (July 1976). "Metabolism of (4-14C)estrone by sheep erythrocytes around the time of parturition". Canadian Journal of Biochemistry. 54 (7): 666–669. doi:10.1139/o76-096. PMID 8195.
- ^ Janer G, LeBlanc GA, Porte C (September 2005). "A comparative study on androgen metabolism in three invertebrate species". General and Comparative Endocrinology. 143 (3): 211–221. doi:10.1016/j.ygcen.2005.03.016. PMID 15922341.
- ^ "Androstenedione, Serum". Test Catalog. Mayo Clinic. Retrieved 1 March 2014.
- ^ a b c Leder BZ, Longcope C, Catlin DH, Ahrens B, Schoenfeld DA, Finkelstein JS (February 2000). "Oral androstenedione administration and serum testosterone concentrations in young men". JAMA. 283 (6): 779–82. doi:10.1001/jama.283.6.779. PMID 10683057.
- ^ a b c d e f Brown GA, Vukovich M, King DS (August 2006). "Testosterone prohormone supplements". Medicine and Science in Sports and Exercise. 38 (8): 1451–61. doi:10.1249/01.mss.0000228928.69512.2e. PMID 16888459.
- ^ a b Badawy MT, Sobeh M, Xiao J, Farag MA (October 2021). "Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences". Molecules. 26 (20): 6210. doi:10.3390/molecules26206210. PMC 8539210. PMID 34684800.
- ^ Sweetman SC, ed. (2009). Martindale: the complete drug reference (36th ed.). London: Pharmaceutical Press. ISBN 978-0-85369-840-1.
- ^ Dohrmann G (9 October 2006). "Is This Dr. Evil?". CNN. Archived from the original on 8 December 2012.
- ^ Rovell D (12 January 2010). "McGwire's Andro Cover Was Very Profitable". CNBC.
- ^ a b c Reents S (2000). Sport and Exercise Pharmacology. Champaign, Ill.: Human Kinetics. ISBN 978-0-87322-937-1.
- ^ Lopez CT (January 2005). "'Andro' supplement off limits in new year". U.S. Air Force Medical Service. Archived from the original on 10 February 2012.
